Antifungal Research, DectiSomes
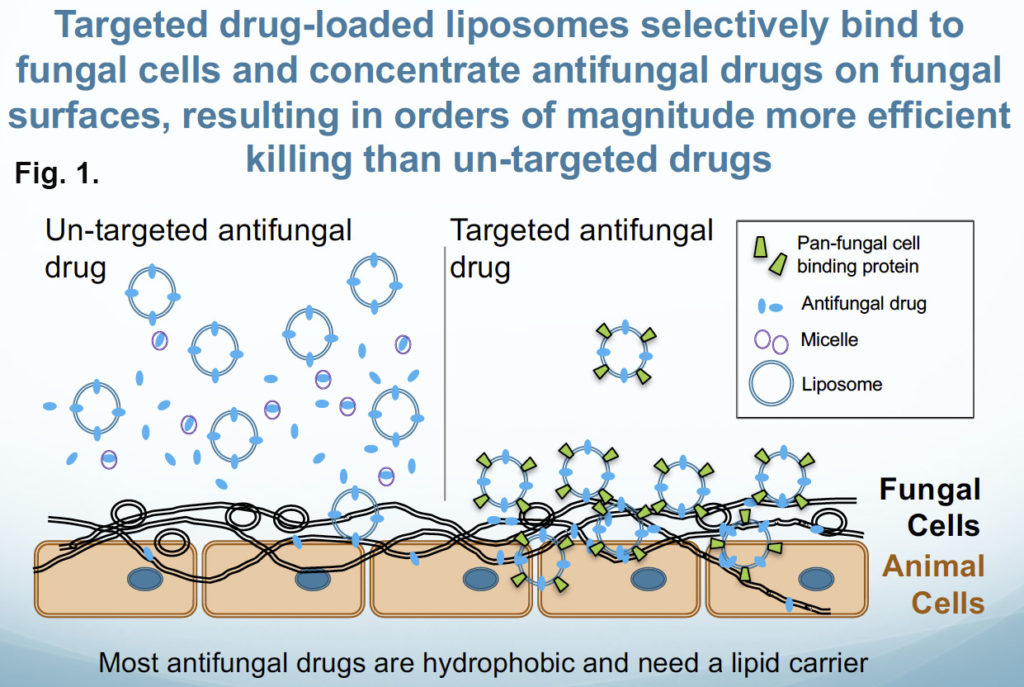
Overview: Along with my colleagues at the University of Georgia, we have designed a new and powerful delivery system for anti-infective drugs that should be applicable to control pathogens from the fungal, animal, bacterial, and protist kingdoms. These targeted liposomal drugs are called DectiSomes. To date, we have explored DectiSomes that direct antifungals specifically to the cell walls and exopolysaccharide matrices of pathogenic fungi1,2,3,4,5,6,7,8,9. Antifungal DectiSomes concentrate antifungal drugs in fungal cells and away from host cells, reduce the effective dose needed to kill and inhibit fungi, and hence, reduces toxicity to mammalian cells, tissues and organs. Targeting is provided by coating the drug-loaded liposomes with fungal binding proteins (Fig. 1)5. To date we have employed the C-type lectin pathogen receptor proteins, Dectin-1, Dectin-2 and DC-SIGN to specifically target drug loaded liposomes to fungal cells. Current antifungal preparations employed in the clinic including the widely used formulation of liposomal Amphotericin B, AmBisome®, do not concentrate the drug on fungal cells and passively deliver it to both fungal cells and host cells.
Need for improved antifungal treatment strategies: Fungal diseases are globally responsible for over 1,350,000 deaths each year. In the U.S. alone, the direct cost of medical treatment for fungal infections is estimated at 7.2 billion dollars annually. Severe invasive fungal infections are potentially fatal with mortality rates ranging from 10% to 95%. The most vulnerable populations include individuals with lung diseases and weakened immune systems. The latter population is growing due to increasing numbers of immunosuppressed and immunocompromised cell and organ transplant patients, patients receiving long-term treatment for inflammatory diseases, and AIDS patients. Hence, annual hospitalization costs to treat invasive fungal infections are expected to rise unless new therapeutic strategies are developed. We have focused our research on three of the four most deadly invasive fungal diseases, aspergillosis, candidiasis, cryptococcosis, and mucormycosis and their most common causitive species, Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans and Rhizopus delemar (a.k.a. R. oryzae, R. arrhizus. Together, each year these disease are responsible globally for >80% of deaths caused by fungal infections and for >4.7 billion dollars in U.S. healthcare costs.
Available antifungal drugs such as amphotericin B, fluconazole, or caspofungin all have serious limitations due to a lack of sufficient fungicidal effect, and/or emerging antifungal resistance, and significant human organ toxicity. Toxicity results from both the high doses and several-month-long duration of antifungal drug therapies needed to clear infections. Amphotericin B was introduced in the 1950s and is still one of the most effective drugs. Its host toxicity issues were moderately reduced when packaged in liposomes (e.g., AmBisome® or AmB-LLs). But the improvement was insufficient to allow the extended or repeated therapies needed to treat many invasive fungal infections without toxic effects. Of the ten antifungals the CDC lists as in common use against the above mentioned pathogens, only one new efficacious drug has been adopted in the last 20 years. The urgent need for improved antifungal therapies is intensified by the recent emergence of fungal pathogens that are resistant to multiple antifungal drugs. In short, these is a critical need for fundamentally improved therapies to treat established and emerging fungal diseases. DectiSome technology can be used to improve the performance of nearly all old and new antifungals.
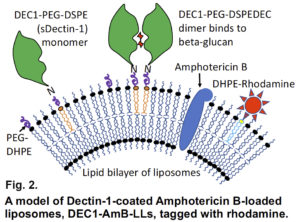
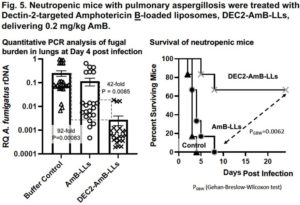
Detailed example of DectiSomes at work. In contrast to animal cells, fungi have a thick outer cell wall and an extracellular exopolysaccharide matrix rich in diverse glucans, mannans, and other oligosaccharides, which are the targets of DectiSomes. Fungal oligoglucan are the cognate ligands of the C-type lectin receptor, Dectin-1. Dectin-1 is a mammalian protein normally expressed on the surface of lymphoid dendritic cells. When Dectin-1 expressed on a dendritic cell binds beta-glucans, these cells signal both innate and adaptive immune responses to infection. We have constructed Amphotericin B loaded liposomes coated with the oligo-glucan binding domain of Dectin-1 (Fig. 2) 5,8. Dectin-1 coated DectiSomes bind to a wide variety of fungal pathogens. We’ve shown that Dectin-1 targeted antifungal liposomes bind to A. fumigatus cells (e.g., germlings and hyphae) and their exopolysaccharide matrix rapidly and with high affinity (Fig. 3) 8, and bind two orders of magnitude more strongly than an untargeted equivalent of the gold standard liposomal drug, AmBisome®. Our Dectin-1- targeted drug-loaded liposomes exhibited more than an order of magnitude greater antifungal activity against A. fumigatus in vitro (Fig. 4)8. Our various other publications show the efficacy of DectiSomes targeted by Dectin-2 and DC-SIGN against C. albicans, A. fumigatus, and C. neoformans 1,3,5,6. Using a neutropenic mouse model of pulmonary aspergillosis, we show that a single treatment with Dectin-2 targeted AmB-loaded liposomes delivering only 0.2 mg/kg AmB (a very low dose) provides order of magnitude reductions in the burden of A. fumigatus cells in the lungs (Fig. 5) and dramatically improves mouse survival, relative to untargeted liposomal AmB2.
- Ambati, S., Pham, T., Lewis, Z.A., Lin, X. & Meagher, R.B. DectiSomes- Glycan Targeting of Liposomal Amphotericin B Improves the Treatment of Disseminated Candidiasis. Antimicrob Agents Chemother 66, 1-13 (2022). https://journals.asm.org/doi/10.1128/AAC.01467-21
- Ambati, S., Ellis, E.C., Pham, T., Lewis, Z.A., Lin, X. & Meagher, R.B. Antifungal Liposomes Directed by Dectin-2 Offer a Promising Therapeutic Option for Pulmonary Aspergillosis. mBio 12, 1-8 (2021). https://www.ncbi.nlm.nih.gov/pubmed/33622715
- Ambati, S., Pham, T., Lewis, Z.A., Lin, X. & Meagher, R.B. DC-SIGN Targets Amphotericin B- Loaded Liposomes to Diverse Pathogenic Fungi. Fungal Biol and Biotech 8, 1-13 (2021). https://rdcu.be/cDPZS
- Meagher, R., Ambati, S., Lewis, Z. & Lin, X. TARGETED NANOPARTICLES AND THEIR USES RELATED TO INFECTIOUS DISEASES. 2021. University of Georgia Research Foundation, I. 63,237,687. 1-93.
Meagher, R., Lewis, Z., Ambati, S. & Lin, X. Aiming for a bull’s-eye: Targeting antifungals to fungi with dectin-decorated liposomes. PLoS Pathog 17, 1-7 (2021). https://www.ncbi.nlm.nih.gov/pubmed/34293050 - Ambati, S., Ellis, E.C., Lin, J., Lin, X., Lewis, Z.A. & Meagher, R.B. Dectin-2-Targeted Antifungal Liposomes Exhibit Enhanced Efficacy. mSphere 4, 1-16 (2019). https://www.ncbi.nlm.nih.gov/pubmed/31666315
- Ambati, S., Ferarro, A.R., Kang, S.E., Lin, J., Lin, X., Momany, M., et al. Dectin-1-Targeted Antifungal Liposomes Exhibit Enhanced Efficacy. mSphere 4, 1-15 (2019). https://www.ncbi.nlm.nih.gov/pubmed/30760610
- Meagher, R.B., Lewis, Z.A., Lin, X., Ambati, S. & Momany, M. Targeted Nanoparticles and Their Uses Related to Fungal Infections. 2019. Patent, U.S. University of Georgia Research Foundation, Inc. (Athens, GA, US). WO/2020/146514, 62/913,489. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020146514&_cid=P11-KHDI99- 36314-1.